Multiple Choice
Write the equilibrium equation for the reverse reaction: 2 CH4(g) + 3 O2(g) ⇌ 2 CO(g) + 4 H2O(g)
A) Kp' = 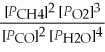
B) Kp' = 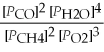
C) Kp' = 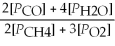
D) Kp' = 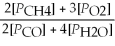
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Which of the following changes in reaction
Q10: The enthalpy for the following reaction is
Q11: If additional SCN<sup>-</sup> is added to the
Q12: For the reaction shown below the value
Q13: The following pictures represent mixtures of A<sub>2</sub>B<sub>4</sub>
Q15: The following pictures represent the equilibrium state
Q16: Which statement about the equilibrium constant is
Q17: According to Le Châtelier's principle,if the volume
Q18: Which of the following is the correct
Q19: Consider the interconversion of A molecules (shaded