Multiple Choice
Consider the interconversion of A molecules (shaded spheres) and B molecules (unshaded spheres) according to the reaction A ⇌ B.Each of the following series of pictures represents a separate experiment in which time increases from left to right. 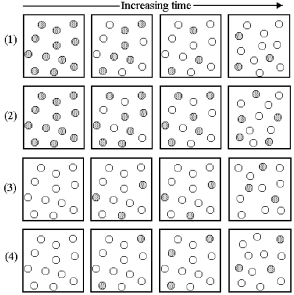
-Which of these experiments has resulted in an equilibrium state?
A) all of the experiments except experiment (1)
B) all of the experiments except experiment (2)
C) all of the experiments except experiment (3)
D) all of the experiments except experiment (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Write the equilibrium equation for the reverse
Q15: The following pictures represent the equilibrium state
Q16: Which statement about the equilibrium constant is
Q17: According to Le Châtelier's principle,if the volume
Q18: Which of the following is the correct
Q20: The esterification of acetic acid and ethanol
Q21: At an elevated temperature,K<sub>p</sub> = 4.2 ×
Q22: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q23: Given the reaction: 2 HI ⇌ H<sub>2</sub>
Q24: The reaction A<sub>2</sub> + B<sub>2 </sub>⇌ 2AB