Multiple Choice
What is the equilibrium equation for the dissociation of formic acid in water? HCOOH (aq) + H2O (l) ⇌ H3O+ (aq) + HCOO- (aq)
A) Kc = 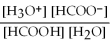
B) Kc = 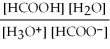
C) Kc = 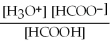
D) Kc = 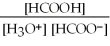
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: The equilibrium constant is equal to 5.00
Q34: What is the equilibrium equation for the
Q35: Which will alter the composition of an
Q36: The following pictures represent the equilibrium state
Q37: Which of the following statements about a
Q39: Write the equilibrium equation for the forward
Q40: At a certain temperature,bromine and nitric oxide
Q41: An equilibrium mixture of CO,O<sub>2</sub> and CO<sub>2</sub>
Q42: What is the equilibrium equation for the
Q43: Phosphorus pentachloride decomposes to phosphorus trichloride and