Multiple Choice
What is the equilibrium equation for the following reaction? C2H4 (g) + 3 O2 (g) ⇌ 2 CO2 (g) + 2 H2O (l)
A) Kp = 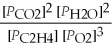
B) Kp = 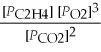
C) Kp = 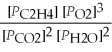
D) Kp = 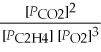
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Given the reaction at a certain temperature:
Q30: Cyclohexane,C<sub>6</sub>H<sub>12</sub>,undergoes a molecular rearrangement in the presence
Q31: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q33: The equilibrium constant is equal to 5.00
Q35: Which will alter the composition of an
Q36: The following pictures represent the equilibrium state
Q37: Which of the following statements about a
Q38: What is the equilibrium equation for the
Q39: Write the equilibrium equation for the forward
Q55: For the isomerization reaction: butane ⇌ isobutane<br>K<sub>p</sub>