Multiple Choice
Find the equilibrium constant for the reaction: A(g) + B(g) ⇌ 2C(g) at 25°C when k equals 1.4 × 10-12 M-1s-1 for the reaction A(g) + B(g) → 2C(g) at 25°C and k equals 2.7 × 10-13 M-1s-1 for the reaction: 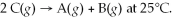
A) 3.8 × 10-25
B) 1.7 × 10-12
C) 1.1 × 10-12
D) 5.2
Correct Answer:

Verified
Correct Answer:
Verified
Q154: Which of the following will result in
Q155: Picture (1)represents an equilibrium mixture of solid
Q156: Picture (1)represents an equilibrium mixture of solid
Q157: What is the equilibrium equation for the
Q158: A crude type of disappearing ink is
Q160: For the reaction 2 A + B<sub>2</sub>
Q161: Acids donate protons to water according to
Q162: What is the equilibrium constant,K<sub>c</sub>,for the reaction:
Q163: Write the equilibrium equation for the forward
Q164: Nitric oxide reacts with oxygen to form