Multiple Choice
Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3. 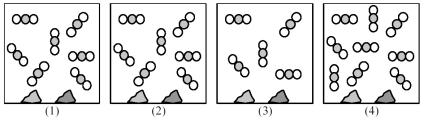
-Which picture (2) -(4) represents the equilibrium mixture when more solid CaCO3 is added?
A) picture (2)
B) picture (3)
C) picture (4)
D) All of these
Correct Answer:

Verified
Correct Answer:
Verified
Q150: Picture (1)represents an equilibrium mixture of solid
Q151: The reaction A<sub>2</sub> + B<sub>2</sub> ⇌ 2
Q152: Shown below is a concentration vs.time plot
Q153: At 25°C,a certain first order reaction has
Q154: Which of the following will result in
Q156: Picture (1)represents an equilibrium mixture of solid
Q157: What is the equilibrium equation for the
Q158: A crude type of disappearing ink is
Q159: Find the equilibrium constant for the reaction:
Q160: For the reaction 2 A + B<sub>2</sub>