Multiple Choice
Shown below is a concentration vs.time plot for the reaction A ⇌ B.For this reaction the value of the equilibrium constant is 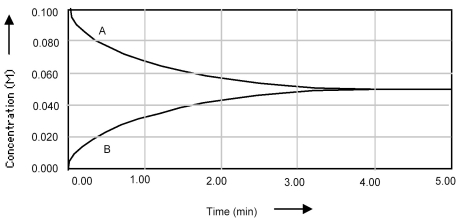
A) Kc < 1.
B) Kc = 0.
C) Kc = 1.
D) Kc > 1.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q142: For a homogeneous equilibrium of gases,which of
Q143: Shown below is a concentration vs.time plot
Q144: The solubility of 1:1 salts is measured
Q145: Given the hypothetical reaction: 2 A(s)+ x
Q146: A 1.50 L vessel contains an equilibrium
Q148: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q149: "If a stress is applied to a
Q150: Picture (1)represents an equilibrium mixture of solid
Q151: The reaction A<sub>2</sub> + B<sub>2</sub> ⇌ 2
Q152: Shown below is a concentration vs.time plot