Multiple Choice
The reaction A2 + B2 ⇌ 2 AB has an equilibrium constant Kc = 1.8.The following pictures represent reaction mixtures that contain A2 molecules (shaded) and B2 molecules (unshaded) ,and AB molecules.Which reaction mixture is at equilibrium? 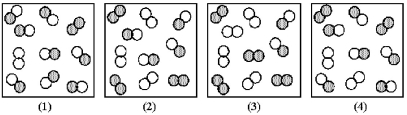
A) reaction mixture (1)
B) reaction mixture (2)
C) reaction mixture (3)
D) reaction mixture (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q146: A 1.50 L vessel contains an equilibrium
Q147: Shown below is a concentration vs.time plot
Q148: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q149: "If a stress is applied to a
Q150: Picture (1)represents an equilibrium mixture of solid
Q152: Shown below is a concentration vs.time plot
Q153: At 25°C,a certain first order reaction has
Q154: Which of the following will result in
Q155: Picture (1)represents an equilibrium mixture of solid
Q156: Picture (1)represents an equilibrium mixture of solid