Multiple Choice
Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3. 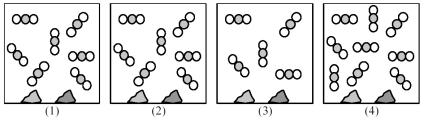
-Which picture (2) -(4) represents the equilibrium mixture when a catalyst is added?
A) picture (2)
B) picture (3)
C) picture (4)
D) All of these
Correct Answer:

Verified
Correct Answer:
Verified
Q107: If K<sub>c</sub> = 2.0 × 10<sup>33</sup> at
Q108: The equilibrium constant,K<sub>p</sub>,equals 3.40 at 25°C for
Q109: K<sub>c</sub> = 1.2 × 10<sup>-42 </sup>at 500
Q110: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic at
Q111: In a reversible reaction,when the rate of
Q113: The reaction A<sub>2</sub> + B<sub>2 </sub>⇌ 2AB
Q114: Ammonium bromide is a crystalline solid that
Q115: A reaction in which reactants form products
Q116: Which of the following statements is false
Q117: The following picture represents the equilibrium state