Multiple Choice
Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3. 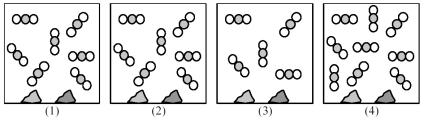
-Which picture (2) -(4) represents the equilibrium mixture after addition of four more CO2 molecules?
A) picture (2)
B) picture (3)
C) picture (4)
D) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Q130: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q131: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q132: At 1000 K,K<sub>p</sub> = 19.9 for the
Q133: What is the equilibrium equation for the
Q134: Consider the reaction A + B ⇌
Q136: For the reaction: 4 HCl(g)+ O<sub>2</sub>(g)⇌ 2
Q137: If K<sub>c</sub> equals 0.110 at 25°C for
Q138: For the reaction H<sub>2</sub>(g)+ S(s)⇌ H<sub>2</sub>S(g),if the
Q139: For the reaction shown below,which change in
Q140: Which statement is true for a reaction