Multiple Choice
The following pictures represent equal volumes of aqueous solutions of three acids HA (A = X,Y,or Z) ;water molecules have been omitted for clarity. 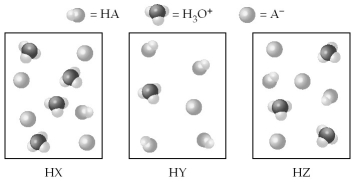
-Arrange the acids in order of increasing value of Ka.
A) Ka(HZ) < Ka(HY) < Ka(HX)
B) Ka(HY) < Ka(HZ) < Ka(HX)
C) Ka(HZ) < Ka(HX) < Ka(HY)
D) Ka(HX) < Ka(HZ) < Ka(HY)
Correct Answer:

Verified
Correct Answer:
Verified
Q210: Calculate the pH of a 0.200 M
Q211: Aniline, (C<sub>6</sub>H<sub>5</sub>NH<sub>2</sub>,K<sub>b</sub> = 4.3 × 10<sup>-10</sup> at
Q212: The following pictures represent solutions of three
Q213: Which one of the following is not
Q214: The following pictures represent equal volumes of
Q216: One way to prepare a solution with
Q217: What is the equilibrium constant expression (K<sub>a</sub>)for
Q218: When dissolved in water,which of the following
Q219: Determine the ammonia concentration of an aqueous
Q220: The following pictures represent aqueous solutions of