Multiple Choice
The following pictures represent aqueous solutions of three acids HA (A = X,Y,or Z) ;water molecules have been omitted for clarity. 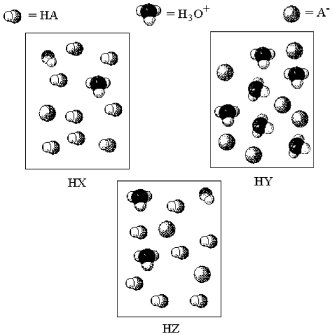
-Which acid solution has the lowest pH?
A) HX
B) HY
C) HZ
D) All have the same pH.
Correct Answer:

Verified
Correct Answer:
Verified
Q215: The following pictures represent equal volumes of
Q216: One way to prepare a solution with
Q217: What is the equilibrium constant expression (K<sub>a</sub>)for
Q218: When dissolved in water,which of the following
Q219: Determine the ammonia concentration of an aqueous
Q221: Dihydrogen phosphate H<sub>2</sub>PO<sub>4</sub><sup>-</sup>,has an acid dissociation constant
Q222: What is the pH of a solution
Q223: Bromothymol blue indicator changes color from yellow
Q224: What is the hydronium ion concentration of
Q225: What is the hydroxide ion concentration and