Multiple Choice
What is the hydroxide ion concentration and the pH for a hydrochloric acid solution that has a hydronium ion concentration of 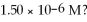
A) 6.67 × 10-8 M,6.82
B) 6.67 × 10-8 M,7.18
C) 6.67 × 10-9 M,5.82
D) 6.67 × 10-9 M,8.17
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q131: What is the pH of a solution
Q132: What is the pH of a solution
Q133: What is the hydroxide ion concentration of
Q134: Bromothymol blue indicator changes color from yellow
Q136: What is the equilibrium constant expression (K<sub>a</sub>)for
Q137: What is the hydronium ion concentration and
Q138: What is the weakest acid among the
Q139: Calculate the pH for an aqueous solution
Q140: The following pictures represent solutions of three
Q196: In the following reaction the unshaded spheres