Multiple Choice
What is the hydronium ion concentration of a 0.200 M hypochlorous acid solution with 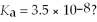 The equation for the dissociation of hypochlorous acid is: HOCl(aq) + H2O(l) ⇌ H3O+(aq) + OCl-(aq) .
The equation for the dissociation of hypochlorous acid is: HOCl(aq) + H2O(l) ⇌ H3O+(aq) + OCl-(aq) .
A) 1.9 × 10-4 M
B) 8.4 × 10-4 M
C) 3.7 × 10-5 M
D) 8.4 × 10-5 M
Correct Answer:

Verified
Correct Answer:
Verified
Q219: Determine the ammonia concentration of an aqueous
Q220: The following pictures represent aqueous solutions of
Q221: Dihydrogen phosphate H<sub>2</sub>PO<sub>4</sub><sup>-</sup>,has an acid dissociation constant
Q222: What is the pH of a solution
Q223: Bromothymol blue indicator changes color from yellow
Q225: What is the hydroxide ion concentration and
Q226: The following pictures represent aqueous solutions of
Q227: Determine the ammonia concentration of an aqueous
Q228: If the ionization constant of water,K<sub>w</sub>,at 40°C
Q229: What are the Br∅nsted-Lowry bases in the