Multiple Choice
Calculate the solubility (in g/L) of calcium fluoride in water at 25°C if the Ksp for Ca F2 is 3.5 × 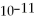 .
.
A) 9.6 × 10-4 g/L
B) 2.1 × 10-4 g/L
C) 3.3 × 10-2 g/L
D) 4.1 × 10-2 g/L
Correct Answer:

Verified
Correct Answer:
Verified
Q78: What is the pH of a solution
Q79: Which statement about buffers is true?<br>A)Buffers have
Q80: Which set of ions precipitate as sulfides?<br>A)Ag<sup>+</sup>,Pb<sup>2+</sup>,Mn<sup>2+</sup><br>B)Pb<sup>2+</sup>,Fe<sup>2+</sup>,Ca<sup>2+</sup><br>C)Co<sup>2+</sup>,Ba<sup>2+</sup>,K<sup>+</sup><br>D)NH<sub>4</sub><sup>+</sup>,Na<sup>+</sup>,K<sup>+</sup>
Q81: What is the approximate pH at the
Q82: What is the approximate pH at the
Q84: 0.10 M potassium chromate is slowly added
Q85: What is the pH of a buffered
Q86: The following pictures represent solutions that contain
Q87: What is the least soluble salt of
Q88: What is the molar solubility of lead(II)chromate