Multiple Choice
Use the graphs below to answer the following questions. 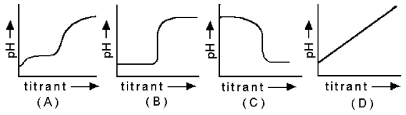
-What is the characteristic pH-titrant curve for the titration of a strong base by a strong acid?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Q61: Selenous acid,H<sub>2</sub>SeO<sub>3</sub> has acid dissociation constants K<sub>a1</sub>
Q62: What is the pH of the resulting
Q63: Potassium chromate is slowly added to a
Q64: What is the pH of a solution
Q65: What is the equilibrium constant expression for
Q67: Consider a buffered solution consisting of H<sub>2</sub>CO<sub>3</sub>
Q68: What is the molar solubility of Mg(OH)<sub>2</sub>
Q69: Oxalic acid,H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> has acid dissociation constants K<sub>a1</sub>
Q70: The following plot shows a titration curve
Q71: CaF<sub>2</sub> has K<sub>sp</sub> = 3.5 × 10<sup>-11</sup>.If