Multiple Choice
The following pictures represent solutions of AgCl,which may also contain ions other than Ag+ and Cl- which are not shown.Gray spheres represent Ag+ ions and dotted spheres represent Cl- ions. 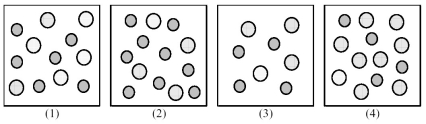
-If solution (1) is a saturated solution of AgCl,which of solutions (1) -(4) represents the solution after a small amount of HCl is added and equilibrium is restored?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q46: TRIS {(HOCH<sub>2</sub>)<sub>3</sub>CNH<sub>2</sub>} is one of the most
Q47: What is the pH of a solution
Q48: In which of the following solutions would
Q49: Which is a net ionic equation for
Q50: Use the graphs below to answer the
Q52: The following plot shows two titration curves,each
Q53: At what pH is the amino acid
Q54: The following pictures represent solutions of CuS,which
Q55: Addition of 0.0125 mol HCl to 150
Q56: What is the pH at the equivalence