Multiple Choice
What is the molarity of a potassium triiodide solution,KI3(aq) ,if 30.00 mL of the solution is required to completely react with 25.00 mL of a 0.200 M thiosulfate solution,Na2S2O3(aq) ? The chemical equation for the reaction is: 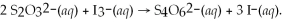
A) 0.0833 M
B) 0.120 M
C) 0.167 M
D) 0.333 M
Correct Answer:

Verified
Correct Answer:
Verified
Q196: The chlor-alkali industry is based on the
Q197: Consider the following standard reduction potentials, Zn<sup>2+</sup>(aq)+
Q198: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Using Table 17.1,find
Q199: What is the Al<sup>3+</sup>:Ag<sup>+</sup>concentration ratio in the
Q200: The following cell has a potential of
Q202: Aluminum requires relatively little protection from corrosion
Q203: A constant current is passed through a
Q204: For a dead battery,<br>A)E is positive and
Q205: At 25°C,E° = +1.88 V for a
Q206: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"