Multiple Choice
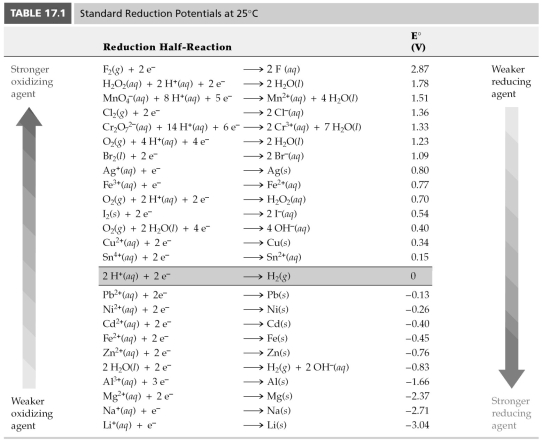
-Using Table 17.1,find E° for 2 H2O(l) → 2 H2(g) + O2(g) .
A) -2.06 V
B) -1.23 V
C) -0.80 V
D) -0.40 V
Correct Answer:

Verified
Correct Answer:
Verified
Q193: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -According to Table
Q194: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q195: What is the relation between ΔG° and
Q196: The chlor-alkali industry is based on the
Q197: Consider the following standard reduction potentials, Zn<sup>2+</sup>(aq)+
Q199: What is the Al<sup>3+</sup>:Ag<sup>+</sup>concentration ratio in the
Q200: The following cell has a potential of
Q201: What is the molarity of a potassium
Q202: Aluminum requires relatively little protection from corrosion
Q203: A constant current is passed through a