Multiple Choice
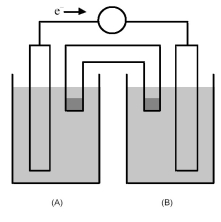
-The cell reaction 2 Fe3+(aq) + Zn(s) → Zn2+(aq) + 2 Fe2+(aq) occurs in the galvanic cell shown above.Which would be the most appropriate choices for the solid electrode in half-cell (A) and in half-cell (B) ?
A) Fe(s) for half-cell (A) and Zn(s) for half-cell (B)
B) Pt(s) for half-cell (A) and Zn(s) for half-cell (B)
C) Fe(s) for half-cell (A) and Fe(s) for half-cell (B)
D) Zn(s) for half-cell (A) and Pt(s) for half-cell (B)
Correct Answer:

Verified
Correct Answer:
Verified
Q166: What is the relationship between the standard
Q167: O<sub>2</sub>(g)+ 4 H<sup>+</sup>(aq)+ 4 e<sup>-</sup>→ 2 H<sub>2</sub>O(l)E°
Q168: Consider the galvanic cell shown below. <img
Q169: Given that E°<sub>red</sub> = -0.26 V for
Q170: In a galvanic cell,the half-reaction H<sub>2</sub>(g)+ 2
Q172: According to the balanced chemical equation 5
Q173: For the galvanic cell that uses the
Q174: Chromium can be electroplated from an aqueous
Q175: Based on the half-reactions and their respective
Q176: Based on the half-reactions and their respective