Multiple Choice
Consider the galvanic cell shown below. 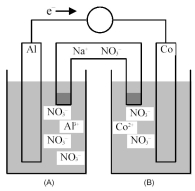
-Identify the anode and cathode,and indicate the direction of Na+ ion and NO3- ion flow from the salt bridge.
A) Al is the anode and Co is the cathode;Na+ ions flow into half-cell compartment (A) and NO3- ions flow into half-cell compartment (B) .
B) Al is the anode and Co is the cathode;NO3- ions flow into half-cell compartment (A) and Na+ ions flow into half-cell compartment (B) .
C) Co is the anode and Al is the cathode;Na+ ions flow into half-cell compartment (A) and NO3- ions flow into half-cell compartment (B) .
D) Co is the anode and Al is the cathode;NO3- ions flow into half-cell compartment (A) and Na+ ions flow into half-cell compartment (B) .
Correct Answer:

Verified
Correct Answer:
Verified
Q163: For a galvanic cell that uses the
Q164: Consider the half-reaction: MnO<sub>4</sub><sup>-</sup>(aq)+ 8 H<sup>+</sup>(aq)+ 5
Q165: The two half-reactions that are used in
Q166: What is the relationship between the standard
Q167: O<sub>2</sub>(g)+ 4 H<sup>+</sup>(aq)+ 4 e<sup>-</sup>→ 2 H<sub>2</sub>O(l)E°
Q169: Given that E°<sub>red</sub> = -0.26 V for
Q170: In a galvanic cell,the half-reaction H<sub>2</sub>(g)+ 2
Q171: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -The cell reaction
Q172: According to the balanced chemical equation 5
Q173: For the galvanic cell that uses the