Multiple Choice
Consider the galvanic cell shown below. 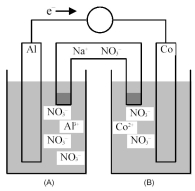
-What is the balanced equation for the cell reaction?
A) 2 Al(s) + 3 Co(s) → 2 Al3+(aq) + 3 Co2+(aq)
B) 2 Al3+(aq) + 3 Co2+(aq) → 2 Al(s) + 3 Co(s)
C) 2 Al(s) + 3 Co2+(aq) → 2 Al3+(aq) + 3 Co(s)
D) 2 Al3+(aq) + 3 Co(s) → 2 Al(s) + 3 Co2+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q108: What is true when the following equation
Q109: Based on the balanced chemical equation shown
Q110: What is the relation between joules (J),volts
Q111: What is the balanced equation for the
Q112: If the concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are varied
Q114: In the relationship ΔG = -nFE°,what is
Q115: Calculate the cell potential at 25°C for
Q116: In a galvanic cell constructed from Pb(s)|
Q117: What is the balanced chemical equation for
Q118: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -For the galvanic