Multiple Choice
Consider the following galvanic cell. 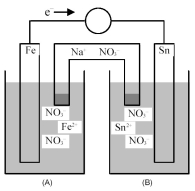
-What is the shorthand notation for the cell?
A) Fe(s) | Fe2+(aq) || Sn(s) | Sn2+(aq)
B) Fe(s) | Fe2+(aq) || Sn2+(aq) | Sn(s)
C) Sn(s) | Sn2+(aq) || Fe(s) | Fe2+(aq)
D) Sn(s) | Sn2+(aq) || Fe2+(aq) | Fe(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q68: O<sub>2</sub>(g)+ 4 H<sup>+</sup>(aq)+ 4 e<sup>-</sup> → 2
Q69: A galvanic cell consists of one half-cell
Q70: In the shorthand notation for a galvanic
Q71: Ag<sup>+</sup>(aq)+ e<sup>-</sup> → Ag(s)E° = +0.800 V
Q72: How many moles of electrons,n,are transferred in
Q74: For the hypothetical reaction 3 A +
Q75: For the galvanic cell Pt(s)∣ Sn<sup>2+</sup>(aq),Sn<sup>4+</sup>(aq)∣∣ Cd<sup>2+</sup>(aq)∣
Q76: Based on the half-reactions and their respective
Q77: Consider the galvanic cell shown below. <img
Q78: When suspected drunk drivers are tested with