Multiple Choice
Consider the galvanic cell shown below. 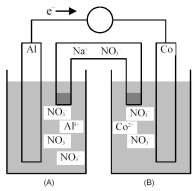
-What is the quantitative change in the cell voltage on increasing the ion concentration in the anode compartment by a factor of 10?
A) +0.03 V
B) +0.02 V
C) -0.02 V
D) -0.03 V
Correct Answer:

Verified
Correct Answer:
Verified
Q72: How many moles of electrons,n,are transferred in
Q73: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q74: For the hypothetical reaction 3 A +
Q75: For the galvanic cell Pt(s)∣ Sn<sup>2+</sup>(aq),Sn<sup>4+</sup>(aq)∣∣ Cd<sup>2+</sup>(aq)∣
Q76: Based on the half-reactions and their respective
Q78: When suspected drunk drivers are tested with
Q79: How many grams of chromium metal are
Q80: NaNO<sub>3</sub>(aq)is employed in the salt bridge.Give the
Q81: The initial concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are both
Q82: What is the molarity of a potassium