Multiple Choice
The initial concentrations of Ag+(aq) and Cu2+(aq) are both 1.0 M.What will happen to the cell voltage if 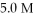 Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
A) decrease.
B) increase.
C) remain the same.
D) can't tell from the information given
Correct Answer:

Verified
Correct Answer:
Verified
Q80: NaNO<sub>3</sub>(aq)is employed in the salt bridge.Give the
Q81: The initial concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are both
Q82: What is the molarity of a potassium
Q83: Shown below is an electrochemical cell with
Q84: Write the overall cell reaction for the
Q86: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Based on the
Q87: A fuel cell is a galvanic cell
Q88: A galvanic cell consists of a Al<sup>3+</sup>/Al
Q89: Which of the following reactions is most
Q90: In the galvanic cell represented by the