Multiple Choice
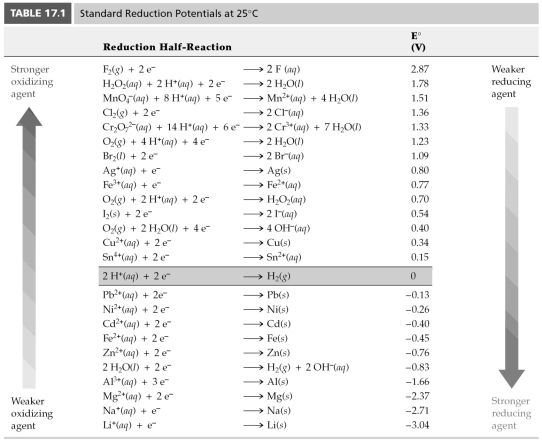
-Based on the following information, Cl2(g) + 2 e- → 2 Cl-(aq) E° = 1.36 V
Zn2+(aq) + 2 e- → 2 Zn(s) E° = -0.76 V
Which of the following chemical species is the strongest reducing agent?
A) Cl2(g)
B) Zn2+(aq)
C) Cl-(aq)
D) Zn(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q81: The initial concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are both
Q82: What is the molarity of a potassium
Q83: Shown below is an electrochemical cell with
Q84: Write the overall cell reaction for the
Q85: The initial concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are both
Q87: A fuel cell is a galvanic cell
Q88: A galvanic cell consists of a Al<sup>3+</sup>/Al
Q89: Which of the following reactions is most
Q90: In the galvanic cell represented by the
Q91: Based on the half-reactions and their respective