Multiple Choice
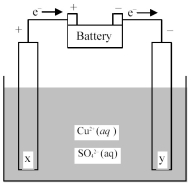
-Is the cell shown above a galvanic or an electrolytic cell? What is the direction of ion flow?
A) Electrolytic cell;Cu2+ ions flow toward electrode x and SO42- ions flow toward electrode y.
B) Electrolytic cell;Cu2+ ions flow toward electrode y and SO42- ions flow toward electrode x.
C) Galvanic cell;Cu2+ ions flow toward electrode x and SO42- ions flow toward electrode y.
D) Galvanic cell;Cu2+ ions flow toward electrode y and SO42- ions flow toward electrode x.
Correct Answer:

Verified
Correct Answer:
Verified
Q49: What is the balanced equation for the
Q50: Which statement is true?<br>A)The cathode is positive
Q51: In order for a cell potential to
Q52: Reduction half-reactions with corresponding standard half-cell potentials
Q53: If a constant current of 1.50 ×
Q55: The cell reaction for a dry cell
Q56: For a particular battery based on one
Q57: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Use Table 17.1
Q59: An electrolytic cell is<br>A)a battery.<br>B)a cell in
Q107: What is the shorthand notation that represents