Multiple Choice
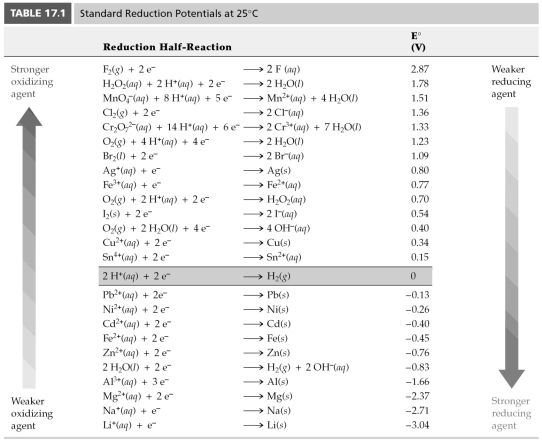
-Use Table 17.1 to determine which of the following is the best oxidizing agent.
A) Fe3+
B) Cl2
C) MnO4-
D) Zn2+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q52: Reduction half-reactions with corresponding standard half-cell potentials
Q53: If a constant current of 1.50 ×
Q54: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Is the cell
Q55: The cell reaction for a dry cell
Q56: For a particular battery based on one
Q59: An electrolytic cell is<br>A)a battery.<br>B)a cell in
Q60: How many grams of chromium metal are
Q61: What is the reduction half-reaction for the
Q62: The standard potential for the following galvanic
Q107: What is the shorthand notation that represents