Multiple Choice
Shown below is an electrochemical cell with anode a and cathode c.Both the anode and the cathode are inert electrodes.The liquid shown in compartment b of the cell is molten potassium chloride,KCl(l) . 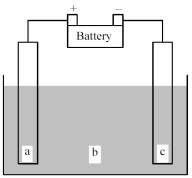
-Determine whether this is a galvanic or an electrolytic cell and give the reaction occurring at the anode and the reaction occurring at the cathode.
A) Electrolytic cell
Anode reaction: K(s) → K+(l) + e-
Cathode reaction: Cl2(g) + 2e- → 2 Cl-(l)
B) Electrolytic cell
Anode reaction: 2 Cl-(l) → Cl2(g) + 2e-
Cathode reaction: K+(l) + e- → K(s)
C) ..Galvanic cell
Anode reaction: K(s) → K+(l) + e-
Cathode reaction: Cl2(g) + 2e- → 2 Cl-(l)
D) .Galvanic cell
Anode reaction: K(s) → K+(l) + e-
Cathode reaction: Cl2(g) + 2e- → 2 Cl-(l)
Correct Answer:

Verified
Correct Answer:
Verified
Q186: What is the reduction half-reaction for the
Q187: In the redox reaction 2 S<sub>2</sub>O<sub>3</sub><sup>2-</sup>(aq)+ I<sub>3</sub><sup>-</sup>(aq)→
Q188: Based on the following information, F<sub>2</sub>(g)+ 2
Q189: Consider the galvanic cell,Pt(s)∣ H<sub>2</sub>(1 atm)|H<sup>+</sup>(1 M)∣∣
Q190: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q192: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q193: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -According to Table
Q194: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q195: What is the relation between ΔG° and
Q196: The chlor-alkali industry is based on the