Multiple Choice
Consider the following galvanic cell. 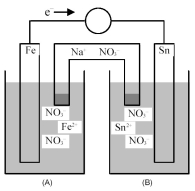
-Identify the anode and cathode,and indicate the direction of ion flow to and from each electrode.
A) Fe is the anode and Sn is the cathode;Fe2+ ions flow to the anode and Sn2+ ions flow from the cathode.
B) Fe is the anode and Sn is the cathode;Sn2+ ions flow to the cathode and Fe2+ ions flow from the anode.
C) Sn is the anode and Al is the cathode;Fe2+ ions flow to the cathode and Sn2+ ions flow from the anode.
D) Sn is the anode and Al is the cathode;Sn2+ ions flow to the anode and Fe2+ ions flow from the cathode.
Correct Answer:

Verified
Correct Answer:
Verified
Q189: Consider the galvanic cell,Pt(s)∣ H<sub>2</sub>(1 atm)|H<sup>+</sup>(1 M)∣∣
Q190: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q191: Shown below is an electrochemical cell with
Q192: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q193: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -According to Table
Q195: What is the relation between ΔG° and
Q196: The chlor-alkali industry is based on the
Q197: Consider the following standard reduction potentials, Zn<sup>2+</sup>(aq)+
Q198: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Using Table 17.1,find
Q199: What is the Al<sup>3+</sup>:Ag<sup>+</sup>concentration ratio in the