Multiple Choice
Consider the reaction shown and identify the statement that is not true.
CaCO3 (s) 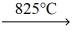
CaO (s) + CO2 (g)
A) This reaction is balanced as written.
B) The reactant must be heated for this reaction to occur.
C) The products are a solid and a gas.
D) Water must be present for this reaction to occur.
E) There are no solutions used in this reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Match the following.<br>-LX(aq)+ MY(aq)→ LY(s)+ MX(aq)<br>A)redox<br>B)acid-base<br>C)precipitation
Q26: What are the spectator ions in the
Q27: The following reaction can be classified as
Q29: Match the following.<br>-HR (aq)+ XOH (aq)→ XR
Q30: Which of the following is always a
Q32: 2 AgNO<sub>3</sub> (aq)+ K<sub>2</sub>SO<sub>4</sub>(aq)→ 2 KNO<sub>3</sub> (aq)+
Q35: In a chemical reaction<br>A)there are equal numbers
Q36: The scientific principle which is the basis
Q36: In the following reaction which species is
Q52: Reduction is the process of<br>A)gaining hydrogen.<br>B)losing oxygen.<br>C)gaining