Multiple Choice
2 AgNO3 (aq) + K2SO4(aq) → 2 KNO3 (aq) + Ag2SO4s)
The net ionic reaction for the balanced equation shown above is
A) Ag+ +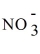
→ AgNO3.
B) 2 K+ +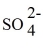
→ K2SO4.
C) K+ +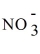
→ KNO3.
D) 2 Ag+ +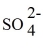
→ Ag2SO4.
E) H+ + OH- → H2O.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Match the following.<br>-LX(aq)+ MY(aq)→ LY(s)+ MX(aq)<br>A)redox<br>B)acid-base<br>C)precipitation
Q14: When the reaction shown is correctly balanced,the
Q27: The following reaction can be classified as
Q29: Match the following.<br>-HR (aq)+ XOH (aq)→ XR
Q30: Which of the following is always a
Q31: Consider the reaction shown and identify the
Q35: In a chemical reaction<br>A)there are equal numbers
Q36: The scientific principle which is the basis
Q36: In the following reaction which species is
Q52: Reduction is the process of<br>A)gaining hydrogen.<br>B)losing oxygen.<br>C)gaining