Multiple Choice
C5H5N + H2CO3 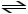
C5H6 N+ + HCO3-
In the reaction shown,the conjugate acid of C5H5N is ________.
A) C5H5N
B) H2CO3
C) C5H6N+
D) HCO3-
E) H3O+
Correct Answer:

Verified
Correct Answer:
Verified
Q28: The pH of a solution with [H<sub>3</sub>O<sup>+</sup>]
Q31: When acids and bases react the product
Q46: Which of the following statements is correct?<br>A)In
Q47: Which reaction best illustrates the behavior of
Q52: Match the following.<br>-conjugate acid<br>A)an acid-base theory that
Q53: The pH of a cup of coffee
Q54: Match the following.<br>-Arrhenius theory<br>A)an acid-base theory that
Q55: Hydrogen cyanide,HCN,is a weak acid.Which equation best
Q81: What is the conjugate acid of HSO<sub>4</sub><sup>-</sup>?<br>A)SO<sub>4</sub><sup>2-</sup><br>B)H<sub>2</sub>SO<sub>4</sub><br>C)H<sub>3</sub>O<sup>+</sup><br>D)OH<sup>-</sup><br>E)H<sub>2</sub>SO<sub>3</sub>
Q86: If the concentration of OH<sup>-</sup> is 1