Multiple Choice
Hydrogen cyanide,HCN,is a weak acid.Which equation best represents its aqueous chemistry?
A) HCN (aq) + H2O (l) 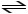
CN- (aq) + H3O+ (aq)
B) HCN (aq) + H2O (l) 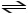
H2CN+ (aq) + OH- (aq)
C) HCN (aq) 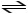
H+ (aq) + CN- (aq)
D) HCN (aq) 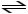
H- (aq) + CN+ (aq)
E) H2O (l) 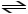
H+ (aq) + OH- (aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q4: What is the normality of a solution
Q22: Which of the following is a weak
Q51: C<sub>5</sub>H<sub>5</sub>N + H<sub>2</sub>CO<sub>3</sub> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4941/.jpg" alt="C<sub>5</sub>H<sub>5</sub>N +
Q52: Match the following.<br>-conjugate acid<br>A)an acid-base theory that
Q53: The pH of a cup of coffee
Q54: Match the following.<br>-Arrhenius theory<br>A)an acid-base theory that
Q67: A buffer solution<br>A)is a salt solution.<br>B)maintains pH
Q81: What is the conjugate acid of HSO<sub>4</sub><sup>-</sup>?<br>A)SO<sub>4</sub><sup>2-</sup><br>B)H<sub>2</sub>SO<sub>4</sub><br>C)H<sub>3</sub>O<sup>+</sup><br>D)OH<sup>-</sup><br>E)H<sub>2</sub>SO<sub>3</sub>
Q86: If the concentration of OH<sup>-</sup> is 1
Q91: Which solution is basic?<br>A)[H<sub>3</sub>O<sup>+</sup>] = 1.0 ×