Multiple Choice
CH3NH2 + HCl 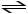
CH3NH3+ + Cl-
A conjugate acid-base pair in the reaction shown is ________ and ________.
A) CH3NH2 and HCl
B) CH3NH2 and Cl-
C) CH3NH3+ and Cl-
D) HCl and Cl-
E) HCl and H3O+
Correct Answer:

Verified
Correct Answer:
Verified
Q5: What is the concentration of an acetic
Q7: The normality of a solution prepared by
Q12: The base forms a new _ bond
Q15: How many mL of 0.100 M NaOH
Q56: Calculate the hydrogen ion concentration in a
Q69: What is the concentration of a phosphoric
Q78: Which of the following is a diprotic
Q85: In an aqueous solution that is acidic,[H<sub>3</sub>O<sup>+</sup>]
Q90: Water and HSO<sub>4</sub><sup>-</sup> can either accept protons
Q94: Which example is not basic?<br>A)shampoo<br>B)vinegar<br>C)window cleaner<br>D)limewater<br>E)Mg(OH)<sub>2</sub>,used in