Multiple Choice
Which of the following structures is likely to exist?
A) 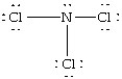
B) 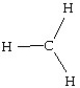
C) 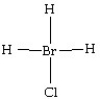
D) 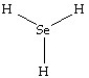
E) None of these may exist.
Correct Answer:

Verified
Correct Answer:
Verified
Q20: A triple bond involves the sharing of
Q21: Based on your knowledge of electronegativities determine
Q22: Match the following.<br>-oxygen difluoride<br>A)ICl<br>B)C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4943/.jpg" alt="Match
Q23: A bond where the electrons are shared
Q24: When two atoms share one or more
Q26: The formula for sulfur hexabromide is<br>A)SF<sub>6.</sub><br>B)SBr<sub>6.</sub><br>C)SBr<sub>4.</sub><br>D)SiBr<sub>6.</sub><br>E)SiB<sub>6.</sub>
Q27: A section of the Periodic Table containing
Q28: For the structure shown,the most likely elements
Q29: If SiCl<sub>4</sub> is named as a covalent
Q30: The smallest possible unit of a covalent