Multiple Choice
Match the following.
-oxygen difluoride
A) ICl
B) C 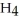
C) N 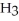
D) PC 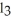
E) 
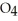
F) CO
G) S 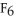
H) S 
I) O 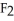
J) Br 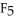
K) CC 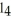
L) I 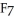
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: Explain how it is possible for CCl<sub>4</sub>
Q18: Which representation of a methane molecule is
Q19: Which of the following structures is the
Q20: A triple bond involves the sharing of
Q21: Based on your knowledge of electronegativities determine
Q23: A bond where the electrons are shared
Q24: When two atoms share one or more
Q25: Which of the following structures is likely
Q26: The formula for sulfur hexabromide is<br>A)SF<sub>6.</sub><br>B)SBr<sub>6.</sub><br>C)SBr<sub>4.</sub><br>D)SiBr<sub>6.</sub><br>E)SiB<sub>6.</sub>
Q27: A section of the Periodic Table containing