Multiple Choice
The phase diagram for a pure compound is shown below .The triple point occurs at 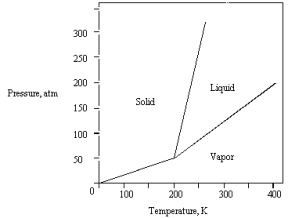
A) 50 atm and 200 K.
B) 0 atm and 200 K.
C) greater than 50 atm and greater than 200 K.
D) 320 atm and 250 K.
E) 200 atm and 400 K.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q181: The phase diagram for a pure compound
Q182: Consider the reaction below:<br>F<sub>2</sub>(g) <span
Q183: How is a reaction quotient defined?<br>A) As
Q184: What is the highest temperature that the
Q185: The osmotic pressure of 1.00 g
Q186: From a plot of Gibbs free
Q187: The vapor pressure of water above
Q189: Consider the following reaction:<br>Ni(CO)<sub>4</sub>(g) <span
Q190: Why is the vapor pressure of cis-dibromoethene
Q191: If the K<sub>sp</sub> of AgI is 1.5