Multiple Choice
What is the highest temperature that the substance shown in the following phase diagram can exist as a liquid? 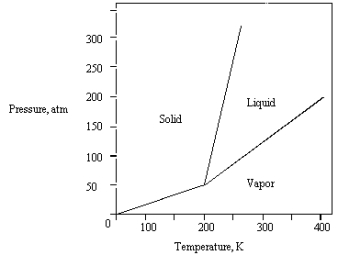
A) any temperature above 200 K
B) 200 K
C) 250 K
D) 350 K
E) 400 K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q179: For a one-component system, at the triple
Q180: If <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q181: The phase diagram for a pure compound
Q182: Consider the reaction below:<br>F<sub>2</sub>(g) <span
Q183: How is a reaction quotient defined?<br>A) As
Q185: The osmotic pressure of 1.00 g
Q186: From a plot of Gibbs free
Q187: The vapor pressure of water above
Q188: The phase diagram for a pure compound
Q189: Consider the following reaction:<br>Ni(CO)<sub>4</sub>(g) <span