Multiple Choice
The phase diagram for a pure substance is given below .
The solid sublimes 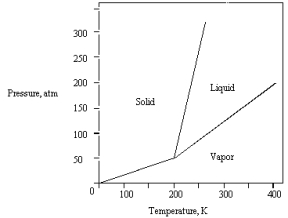
A) at 400 K and 200 atm.
B) at 200 K and 100 atm.
C) at 300 K and 100 atm.
D) at 300 K and 75 atm.
E) if warmed at any pressure below 50 atm.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: Rank the following species in order
Q30: The phase diagram for sulfur is given
Q31: A plot of ln(vapor pressure) versus 1/T
Q32: The normal boiling point of ethanol
Q33: For the decomposition of ammonia to nitrogen
Q35: Consider the phase diagrams for water and
Q36: If a 1-L flask containing D<sub>2</sub>(g),N<sub>2</sub>(g),and ND<sub>3</sub>(g)at
Q37: Consider the following reaction at a
Q38: For the decomposition of ammonia to nitrogen
Q39: The critical temperature of N<sub>2</sub> is <font