Multiple Choice
The phase diagram for sulfur is given below. 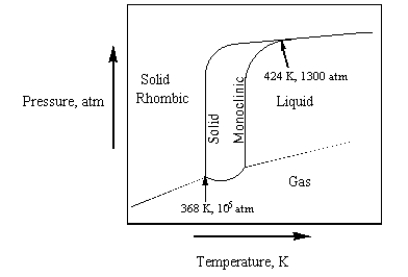 At 424 K and 1300 atm,
At 424 K and 1300 atm,
A) rhombic sulfur, monoclinic sulfur, sulfur liquid, and sulfur gas exist in equilibrium.
B) only rhombic sulfur and sulfur gas exist in equilibrium.
C) rhombic sulfur, monoclinic sulfur, and liquid sulfur exist in equilibrium.
D) only rhombic sulfur is present.
E) only monoclinic sulfur is present.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Calculate the concentration of argon in lake
Q2: In a closed vessel containing water the
Q3: The phase diagram for a pure substance
Q4: What is the molality of CrCl<sub>3</sub> in
Q5: Consider the reaction<br>CO(g)+ 2H<sub>2</sub>(g) <br> <span
Q7: Consider the phase diagram for CO<sub>2</sub>
Q8: The vapor pressure of benzene at
Q9: What is the molality of carbon tetrachloride
Q10: The freezing point of seawater is
Q11: At the normal boiling point of chlorine,<br>238.5