Multiple Choice
The phase diagram for a pure substance is given below. 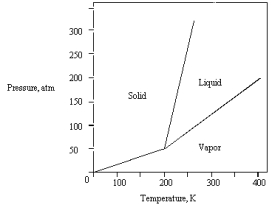 What pressure must be applied to liquefy a sample at 425 K?
What pressure must be applied to liquefy a sample at 425 K?
A) 350 atm
B) The sample cannot be liquefied at 425 K.
C) 150 atm
D) 50 atm
E) 250 atm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Calculate the concentration of argon in lake
Q2: In a closed vessel containing water the
Q4: What is the molality of CrCl<sub>3</sub> in
Q5: Consider the reaction<br>CO(g)+ 2H<sub>2</sub>(g) <br> <span
Q6: The phase diagram for sulfur is given
Q7: Consider the phase diagram for CO<sub>2</sub>
Q8: The vapor pressure of benzene at
Q9: What is the molality of carbon tetrachloride
Q10: The freezing point of seawater is
Q11: At the normal boiling point of chlorine,<br>238.5