Multiple Choice
The titration curve for the titration of 0.100 M Na2CO3(aq) with 0.100 M HClO4(aq)
Is: 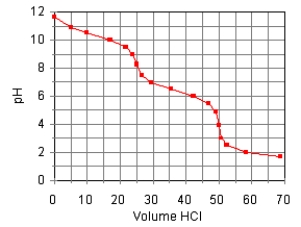
The main species in the solution after the addition of 35 mL of HClO4 are
A) HCO3-, H2CO3, Na+, and ClO4-.
B) H2CO3, Na+, and ClO4-.
C) CO32-, HCO3, Na+, and ClO4-.
D) CO32-, Na+, and ClO4-.
E) HCO3-, Na+, and ClO4-.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Use the following diagram of a
Q14: A buffer contains equal concentrations of NH<sub>3</sub>(aq)
Q15: What is the value of E
Q16: What is the main factor that determines
Q17: Which of the following 0.10 M aqueous
Q19: If <span class="ql-formula" data-value="\alpha"><span class="katex"><span
Q20: For the cell diagram<br>Cd(s)|CdSO<sub>4</sub>(aq)|Hg<sub>2</sub>SO<sub>4</sub>|Hg(l)<br>What reaction occurs
Q21: For NH<sub>3</sub>, pK<sub>b</sub> = 4.74.What is the
Q22: In a working electrochemical cell (+ cell
Q23: The following compounds are available as