Multiple Choice
Use the following diagram of a cell to answer questions 59-64: 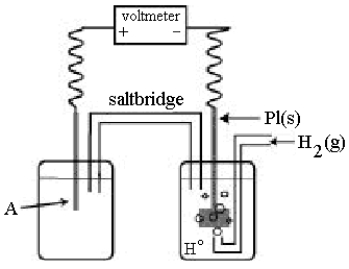
-Using the cell shown above, A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE) .When the voltmeter reading is -0.80 V,
Which half-reaction occurs in the left-hand cell compartment?
A) Ag(s) Ag+(aq) + e-
B) Ag+(aq) + e- Ag(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q8: The conjugate acid of HPO<sub>4</sub><sup>2</sup><sup>-</sup> is<br>A) HPO<sub>4</sub><sup>2</sup><sup>-</sup>.<br>B)
Q9: Which species will oxidize Cr<sup>2+</sup> but not
Q10: Which of the following is the weakest
Q11: True or false: the pH of 0.10
Q12: What is the pH at the half-stoichiometric
Q14: A buffer contains equal concentrations of NH<sub>3</sub>(aq)
Q15: What is the value of E
Q16: What is the main factor that determines
Q17: Which of the following 0.10 M aqueous
Q18: The titration curve for the titration of