Multiple Choice
What is the maximum mass in grams of NH3 that can be produced by the reaction of 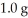 of N2 with
of N2 with 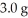 of H2 via the equation below?
of H2 via the equation below?
N2 (g) + H2 (g) → NH3 (g) (not balanced)
A) 2.0
B) 1.2
C) 0.61
D) 17
E) 4.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: What is the mass % of carbon
Q27: The combustion of propane (C<sub>3</sub>H<sub>8</sub>)produces CO<sub>2</sub> and
Q28: When the following equation is balanced,the coefficients
Q29: Combustion of a 1.031-g sample of a
Q30: There are _ mol of carbon atoms
Q32: The formula weight of Zn(ClO<sub>4</sub>)<sub>2</sub> is _
Q33: What is the mass percent of C
Q34: The mass % of C in methane
Q35: Sulfur and fluorine react in a combination
Q36: Of the reactions below,which one is a