Multiple Choice
Sulfur and fluorine react in a combination reaction to produce sulfur hexafluoride: S (s) + 3F2(g) → SF6 (g)
The maximum amount of SF6 that can be produced from the reaction of 32.1 g of sulfur with 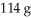 of fluorine is ________ g.
of fluorine is ________ g.
A) 584
B) 438
C) 146
D) 48.7
E) 219
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: There are _ mol of carbon atoms
Q31: What is the maximum mass in grams
Q32: The formula weight of Zn(ClO<sub>4</sub>)<sub>2</sub> is _
Q33: What is the mass percent of C
Q34: The mass % of C in methane
Q36: Of the reactions below,which one is a
Q37: The combustion of ammonia in the presence
Q38: When the following equation is balanced,the coefficient
Q39: Calculate the percentage by mass of lead
Q40: What is the empirical formula of a