Multiple Choice
The value of ΔH° for the reaction below is +128.1 kJ: CH3OH (l) → CO (g) + 2H2 (g)
How many kJ of heat are consumed when 15.5 g of C 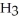 OH (l) decomposes as shown in the equation?
OH (l) decomposes as shown in the equation?
A) 0.48 kJ
B) 62.0 kJ
C) 1.3 × 102 kJ
D) 32 kJ
E) 8.3 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: The value of ΔE for a system
Q6: Calculate the value of ΔE in joules
Q7: Work equals mass times distance.
Q8: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q9: Energy units include watts,volts,and newtons.
Q11: The value of ΔH° for the reaction
Q12: Given the data in the table below
Q13: A sample of iron absorbs 81.0 J
Q14: A _ ΔH corresponds to an _
Q15: The specific heat capacity of methane gas