Multiple Choice
Given the data in the table below and ΔH°rxn for the reaction SO2Cl2 (g) + 2H2O (l) → H2SO4 (l) + 2HCl (g) ΔH° = -62 kJ
ΔH°f of HCl (g) is ________ kJ/mol. 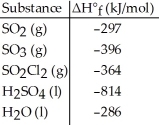
A) -184
B) 60
C) -92
D) 30
E) Insufficient data are given.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Work equals mass times distance.
Q8: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q9: Energy units include watts,volts,and newtons.
Q10: The value of ΔH° for the reaction
Q11: The value of ΔH° for the reaction
Q13: A sample of iron absorbs 81.0 J
Q14: A _ ΔH corresponds to an _
Q15: The specific heat capacity of methane gas
Q16: Hydrogen gas and bromine gas react to
Q17: The internal energy of a system is