Multiple Choice
Given the data in the table below,ΔH°rxn for the reaction 2SO2 (g) + O2 (g) → 2SO3 (g)
Is ________ kJ. 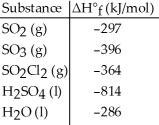
A) -99
B) 99
C) -198
D) 198
E) The ΔH°f of O2 (g) is needed for the calculation.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q39: The primary component of natural gas is
Q40: _ is defined as the energy used
Q41: All of the following are considered fossil
Q42: Given the following reactions 2S (s)+ 3O<sub>2</sub>
Q43: Given the following reactions N<sub>2</sub> (g)+ 2O<sub>2</sub>
Q45: In the presence of excess oxygen,methane gas
Q46: The term Btu which stands for _
Q47: How much heat is required to raise
Q48: A 100-watt electric incandescent light bulb consumes
Q49: The value of ΔH° for the reaction