Multiple Choice
How much heat is required to raise the temperature of a 1.15 kg piece of copper metal from 25.0 °C to 77.5 °C? The specific heat capacity of solid copper metal is 0.385 J/g-K.
A) 2.32 × 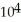 J
J
B) 1.57 × 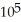 J
J
C) 23.2 J
D) 6.38 × 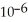 J
J
E) 0.00638 J
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Given the following reactions 2S (s)+ 3O<sub>2</sub>
Q43: Given the following reactions N<sub>2</sub> (g)+ 2O<sub>2</sub>
Q44: Given the data in the table below,ΔH°<sub>rxn</sub>
Q45: In the presence of excess oxygen,methane gas
Q46: The term Btu which stands for _
Q48: A 100-watt electric incandescent light bulb consumes
Q49: The value of ΔH° for the reaction
Q50: The temperature of a 15-g sample of
Q51: Given the following reactions N<sub>2</sub> (g)+ O<sub>2</sub>
Q52: A typical fast food meal consists of